About Hackensack Meridian Health
Hackensack Meridian Health nurses care for patients and their families at every stage of life, 24 hours a day, at bedsides in 9 community hospitals, 4 academic medical centers, a behavioral health hospital, 2 children's hospitals, 2 rehabilitation hospitals, medical offices, and care facilities across the state.
Our team members are the heart of what makes us better. At Hackensack Meridian Health we help our patients live better, healthier lives — and we help one another to succeed. With a culture rooted in connection and collaboration, our employees are team members. Here, competitive benefits are just the beginning. It’s also about how we support one another and how we show up for our community. Together, we keep getting better - advancing our mission to transform healthcare and serve as a leader of positive change.
Hackensack Meridian Health is one of the most recognized health care networks in the country for nursing excellence with 7 Magnet designations. In joining our nursing team, you'll work with collaborative colleagues, who are pushing each other - and patient care - to keep getting better.
Success Profile
What makes a successful Lead Clinical Research Nurse - JTCC - Oncology, Lymphoma - F/T Days at Hackensack Meridian Health? Check out the top traits we're looking for and see if you have the right mix.
- Collaborative
- Courageous
- Compassionate
- Creative
- Connected
Job Description
- Requisition # 2024-156580
- Shift Day
- Status Full Time with Benefits
Overview
Our team members are the heart of what makes us better. At Hackensack Meridian Health we help our patients live better, healthier lives — and we help one another to succeed. With a culture rooted in connection and collaboration, our employees are team members. Here, competitive benefits are just the beginning. It’s also about how we support one another and how we show up for our community. Together, we keep getting better - advancing our mission to transform healthcare and serve as a leader of positive change.
The Lead Clinical Research Nurse is responsible for providing guidance, training, and coordination of the assigned oncology research divisions clinical research nursing team. This role plays a pivotal role in coordinating and overseeing clinical operations of all assigned Clinical Research Nurse and Clinical Trials and participates in assessing, planning, implementing and evaluating Compliant Patient Care in clinical research studies. This role works under the general supervision of the Principal Investigator(s) and Oncology Clinical Research Administration.
Responsibilities
A day in the life of a Lead Clinical Research Nurse at Hackensack Meridian Health may include:
- Works together with and oversees all assigned Clinical Research Nurse to:
- Assures that all protocol revisions, informed consents, continuing reviews, serious adverse events are submitted to the appropriate IRB of record in a timely manner.
- Recruits and evaluates potential study patients, and works with clinical research coordinator(s) to schedule required appointments and interviews.
- Identifies the needs of the patient population served and modify and deliver care that is specific to those needs (i.e., age, culture, hearing and/or visually impaired, etc.). This process includes communicating with the patient, parent, and/or primary caregiver(s) at their level (developmental/age, educational, literacy, etc.).
- Reviews medical records for potential study patients and ensures that medical records include documentation of all laboratory test results and procedures and progress of study patients, following guidelines set forth by the protocol sponsors.
- Instructs potential study patients, designated caregiver, physicians, nurse clinicians and other ancillary staff members involved in the care of the patient on aspects of patient's care, available trials, treatments and side effects.
- Assists investigator with consent process assuring study patients understand clinical trials and obtain written informed consent.
- Educates study patients concerning informed consent procedures, HIPAA authorization.
- Documents study patient's medical history including but not limited to past medical/surgical treatments, significant medical conditions, and medication history per protocol guidelines.
- Performs nursing assessments and monitors study patient's progress during clinical trials; Tracks study patient's response by documenting on toxicity flow sheet, medication flow sheet and nurses' progress notes.
- Maintains accurate, complete, up-to-date records on each patient participating in a clinical trial protocol in all applicable systems (i.e. electronic medical record, clinical trial management system, departmental and protocol specific databases).
- Evaluates and develops study patient education materials and gives study patient and/or designated caregiver instructions on drug administration and other medical information; creates study specific calendars for study patients. l. Plans for study patient's appropriate care under the direction of a physician or advanced practice nurse.
- Notifies principal investigator of any adverse events and serious adverse events, including evidence of drug toxicity or unexpected side effects.
- Reports all serious adverse events to sponsor and IRB of record according to established timelines.
- Performs and/or oversee a variety of clinical duties that may include but not limited to: EKGs, processing/shipping of blood serum, urine and communicate results to PI and/or APN. p. Together with the principal investigator, reviews and processes all Safety Reports (INDs, SUGARs) as per institutional policies and procedures.
- Interacts with regulatory specialists and principal investigators and sub-investigators on all regulatory issues and changes within the protocol. 3. In collaboration with the principal investigator, clinical research coordinator, and clinical team, participates in the review of studies for feasibility and evaluates potential competition with other protocols prior to submitting study. 4. Reviews study with principal investigator and/or clinical research coordinator to a budget outlining standard of care and research costs. Finalizes budget draft with budget coordinator.
- Act as principal investigator's representative as appropriate. This may include communicating with sponsors and their representatives, the IRB and other medical personnel.
- Prepares and assists for sponsor monitor site visits and ensures all supporting documentation records are adequate and available for the visit; Meets with monitor at least once during each monitor site visit and resolves all issues found during visit.
- Assists the principal investigator in preparing for publication. Works with analysts and assists with queries related to data to evaluate the significance of collected data.
- Provides education to all departments and clinical areas where study is performed.
- Attends research meetings and conferences as required.
- Coordinates and manages the daily operations of the division in the abscess of administration.
- Participates in staff meetings and in-service education of nursing and medical staff.
- Promotes safe patient care through assessing for patient, family or team member issues.
- Plays an active role in resolution of patient or team member problem; conflict resolution.
- Assures daily schedules within the division to support safe patient care and assists in maintaining appropriate staffing within the division in compliance with acuity, etc.
- Interprets supports and communicates HMH & JTCC policies, standards and procedures.
- Efficiently identifies divisional problems and develops solutions to review with Oncology Research Administration.
- Interacts in an appropriate and timely manner in conflict situations facilitating constructive resolutions and positive outcomes.
- Serves as a role model for all research staff in all aspects of nursing and clinical research conduct (i.e professionalism, quality care).
- Acts as a liaison for research nurses for concerns between departments and facilities and collaborates with the JTCC Management TEam on inter and intra departmental issues that may occur when dealing with other members of the organization.
- Provides employee supervision to further enhance quality care and gives feedback to staff.
- Identifies patient care issues and collaborates with the JTCC Management Team to initiate change.
- Other duties and/or projects as assigned.
- Adheres to HMH Organizational competencies and standards of behavior.
Qualifications
Education, Knowledge, Skills and Abilities Required:
- Graduate of a NLN/AACN accredited program in nursing.
- 3 years of progressive oncology nursing
- 3 years oncology clinical research nursing experience
- Adheres to the American Nurses Association standards
- Strong attention to detail and customer service focus is required
- Excellent communication, organizational, presentation, documentation, and interpersonal skills are required
- Ability to work independently, or in a team, and handle multiple deadline driven tasks in a dynamic environment is essential
- Proficient computer skills that may include but are not limited to Microsoft Office and/or Google Suite platforms
- Reviews and complies with all relevant HUMC and Business Unit policies and procedures, and local, state, and Federal laws and regulations
Education, Knowledge, Skills and Abilities Preferred:
- BSN Preferred
- Mandatory education on human subject research and GCP (CITI Training and Certification)
- Knowledge of clinical trials and the regulation (local, state, and federal) of such.
- Familiarity with basic scientific and healthcare principles and terminology
- Ability to work in a fast-paced environment and manage competing tasks and demands
Licenses and Certifications Required:
- NJ State Professional Registered Nurse License
Licenses and Certifications Preferred:
- Certified Clinical Research Professional (CCRP) and/or Certified Clinical Research Associate (CCRA) and/or Certified Clinical Research Coordinator (CCRC)
HACKENSACK MERIDIAN HEALTH (HMH) IS AN EQUAL OPPORTUNITY EMPLOYER
All qualified applicants will receive consideration for employment without regard to age, race, color, creed, religion, sex, sexual orientation, gender identity or expression, pregnancy, breastfeeding, genetic information, refusal to submit to a genetic test or make available to an employer the results of a genetic test, atypical hereditary cellular or blood trait, national origin, nationality, ancestry, disability, marital status, liability for military service, or status as a protected veteran.
Our Network
Hackensack Meridian Health (HMH) is a Mandatory Influenza Vaccination Facility
All qualified applicants will receive consideration for employment without regard to age, race, color, creed, religion, sex, sexual orientation, gender identity or expression, pregnancy, breastfeeding, genetic information, refusal to submit to a genetic test or make available to an employer the results of a genetic test, atypical hereditary cellular or blood trait, national origin, nationality, ancestry, disability, marital status, liability for military service, or status as a protected veteran.
As a courtesy to assist you in your job search, we would like to send your resume to other areas of our Hackensack Meridian Health network who may have current openings that fit your skills and experience.
Benefits
-
workplace excellence
-
collaborative work environment
-
work-life balance
-
compassionate nursing culture
-
mentoring programs
-
substantial tuition reimbursement
Eric
Gossar Assistant Nurse Manager, Critical Care Unit
Southern Ocean Medical Center
To be a newer nurse and become an assistant manager of a critical care unit speaks to the opportunities Hackensack Meridian Health can provide. Also, the teamwork and team members I have been exposed to here are second to none."
Start Exploring
See the area you’ll be working in and get an idea of what your daily routine around the office can be like.
Learn MoreNURSING ANNUAL REPORT
Check out our latest Nursing Annual Report to learn more about the achievements of our nursing team.
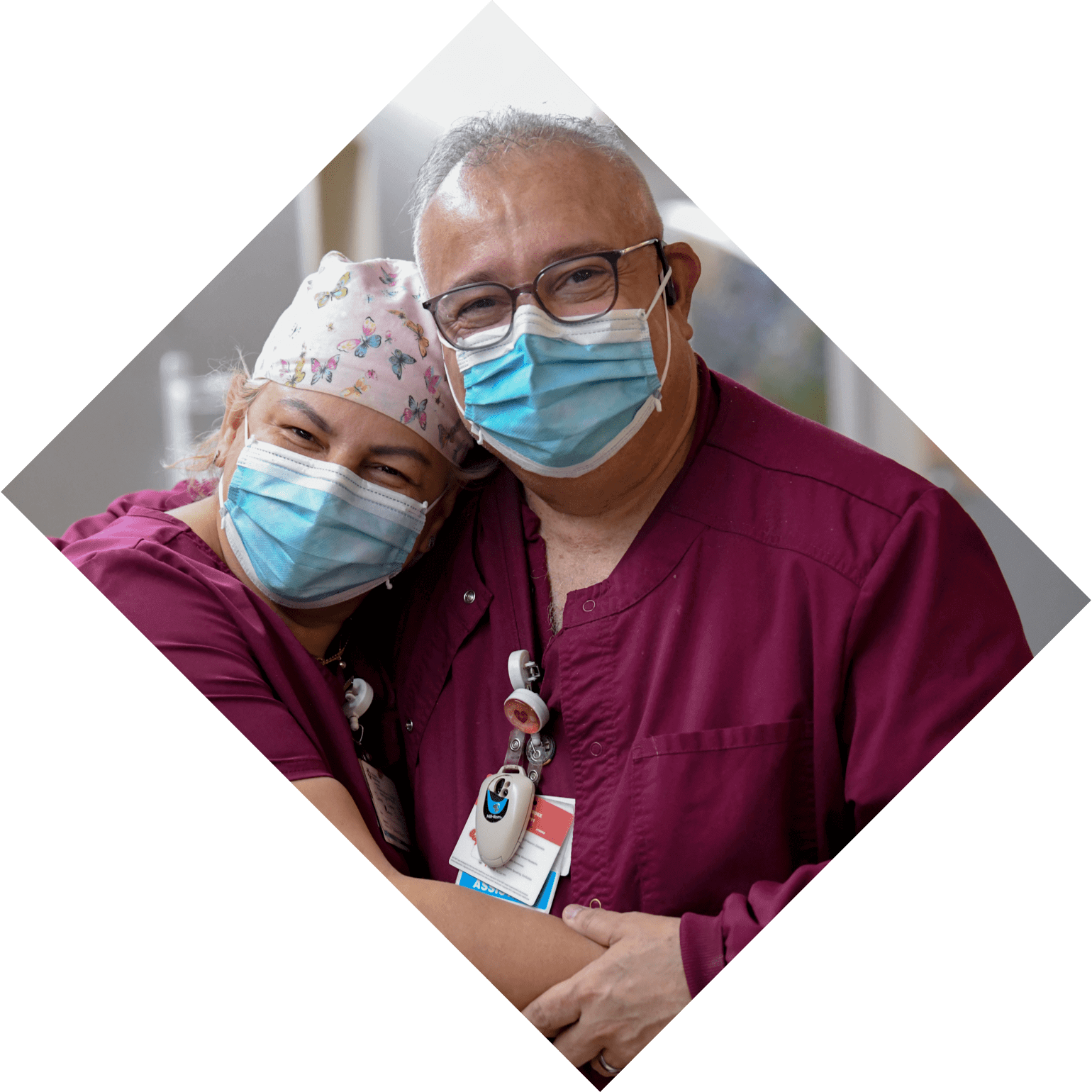

-

Nursing with us
Be part of an exceptional and nationally recognized network, where nurses are ready to provide the care patients need to keep getting better.
Learn More -

Nursing opportunities
At Hackensack Meridian Health, our integrated nursing team is ready to provide the highest level of care each and every day.
Learn More -

EVENTS
Our event opportunities allow teammates alternate ways of meeting and collaborating, which builds a sense of belonging.
Learn More
JOBS FOR YOU
You have no recently viewed jobs
-
Certified Medical Assistant - Cardio - physician Practice -
HMH PHYSICIAN SERVICES, INC. Bayville, New Jersey -
Certified Medical Assistant - Multi-Specialty - Physician Practice
HMH PHYSICIAN SERVICES, INC. Bayville, New Jersey -
Registered Nurse- Blake Detox & Recovery Unit- F/T Evenings
HMH CARRIER CLINIC INC Belle Mead, New Jersey -
Registered Nurse - Comprehensive Services Psych Unit - P/T without Benefits, Days
HMH CARRIER CLINIC INC Belle Mead, New Jersey
STAY CONNECTED
From roles that are right for you to new opportunities, join our talent community and stay up to date with job openings and more.
